More Information
Submitted: January 28, 2022 | Approved: February 16, 2022 | Published: February 18, 2022
How to cite this article: Lombard CM, Li J, Shrestha B. Post-operative agranulocytosis caused by intravenous cefazolin: A case report with a discussion of the pathogenesis. Arch Pathol Clin Res. 2022; 6: 009-012.
DOI: 10.29328/journal.apcr.1001030
Copyright License: © 2022 Lombard CM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Agranulocytosis; Neutropenia; Postoperative; Cephalosporin; Cephazolin; Pathogenesis
Post-operative agranulocytosis caused by intravenous cefazolin: A case report with a discussion of the pathogenesis
Charles M Lombard* , Jiali Li2 and Bijayee Shrestha3
, Jiali Li2 and Bijayee Shrestha3
1Department of Pathology, El Camino Hospital Mountain View, CA 94040, USA
2Division of Hematology and Oncology, Cancer Center, El Camino Hospital Mountain View, CA, USA
3Department of Pathology, El Camino Hospital Mountain View, CA, USA
*Address for Correspondence: Charles M Lombard, MD, Department of Pathology GC # 431, 2500 Grant Road, El Camino Hospital Mountain View, CA 94040, USA, Email: [email protected]
A case of post-operative agranulocytosis which occurred in a 66-year-old woman following surgery for endometrial carcinoma is reported. The agranulocytosis had a rapid onset, being detected on the first post-operative day. The causative agent, cefazolin was given to the patient intraoperatively. The agranulocytosis persisted until the 22nd postoperative day. A bone marrow biopsy performed on post-operative day four showed a left-shifted myeloid maturation pattern but not a maturation arrest. The pathogenesis of drug-induced neutropenia/agranulocytosis is discussed. It is postulated that reversible binding of cefazolin to albumin accounts for the prolonged duration of agranulocytosis.
Drug-induced neutropenia/agranulocytosis is a well-recognized phenomenon [1-4]. The cephalosporin class of drugs is a group of drugs that are frequently implicated as the causative agent of neutropenia/agranulocytosis [5-7]. In most cases, there is a prolonged period of drug exposure prior to developing neutropenia, with an average time reported between 1 and 6 months [2-4]. This prolonged length of exposure is consistent with the need for a hypersensitivity type reaction to the drug to evolve. Although some have suggested that toxic effects on the bone marrow with maturation arrest may contribute to the pathogenesis of the neutropenia [7], most evidence is consistent with antibody-mediated immune pathogenesis [4]. Like the pathogenesis of penicillin-mediated neutropenia [8], the pathogenesis of cephalosporin-associated neutropenia is thought to be secondary to the presence of drug-dependent anti-neutrophil antibodies [6,9]. The duration of drug-induced neutropenia has been reported to be an average of 8 days with a range of 4-24 days [1]. The half-lives of the drugs implicated in the pathogenesis of neutropenia/agranulocytosis have half-lives of much shorter duration compared to the reported lengths of the duration of prolonged neutropenia/agranulocytosis with drug half-lives in the range of hours and not days. The dilemma of how a drug-dependent antibody reaction could persist for up to 24 days has not been addressed.
A 66-year-old woman presented with vaginal bleeding. A subsequent endometrial curettage demonstrated a FIGO grade 1 endometrial adenocarcinoma. The patient was scheduled for a robotic-assisted laparoscopic hysterectomy with bilateral salpingo-oophorectomy with sentinel lymph node biopsies. A pre-operative complete blood count showed a white blood cell count of 6.8 K/uL with 64% neutrophils and an absolute neutrophil count of 4.3 K/uL. The operation was uneventful and the pathologic findings showed an endometrial adenocarcinoma, FIGO grade 1 with no evidence of myometrial invasion. Right and left pelvic sentinel lymph node biopsies were negative for metastatic carcinoma.
On the first postoperative day, the patient developed a fever. A complete blood count showed a white blood cell count of 1.93 K/uL with 0% neutrophils. The review of intraoperative medications revealed an intravenous injection of 2 g cefazolin during the operation.
Other medications administered during the operation included fentanyl, midazolam, propofol, succinylcholine, rocuronium, phenylephrine, glycopyrrolate, neostigmine, metoclopramide, dexamethasone, and ondansetron. Of all medications administered, cefazolin was the one most clearly associated with neutropenia and agranulocytosis. The complete blood count documenting agranulocytosis was taken exactly 34 hours after the intravenous administration of the drug cefazolin.
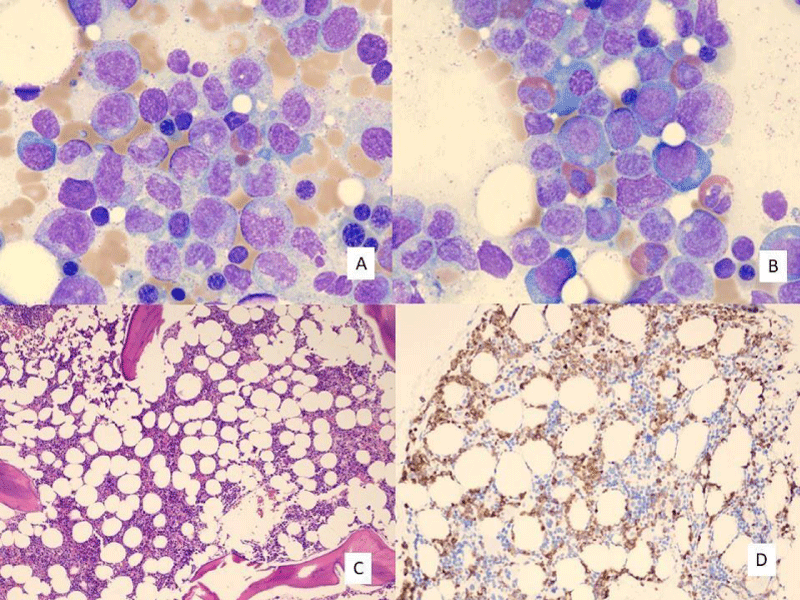
The patient was treated with vancomycin and cefepime. The patient was also administered a 7-day course of the granulocyte colony-stimulating factor granix at 480 mg per day. The patient had a bone marrow biopsy performed 4 days after her operation. The bone marrow biopsy with flow cytometry demonstrated marrow cellularity of an estimated 50%. The myeloid:erythroid ratio was estimated at 2:1. A markedly left-shifted myeloid maturation pattern was noted on the cell count (Table 1) and by flow cytometry. Flow cytometry also demonstrated a mild mature monocytosis (20%) and eosinophilia (11%). Cytogenetic studies and myelodysplasia FISH studies were normal. Bone marrow biopsy findings are demonstrated in Figure 1.
| Table 1: Post-operative day 4 bone marrow aspirate 500 cell differential count. | |
| Blasts: | 3% |
| Promyelocytes: | 6% |
| Myelocytes: | 26% |
| Metamyelocytes: | 7% |
| Bands/Neutrophils: | 3% |
| Monocytes: | 4% |
| Eosinophils: | 6%. |
| Erythroid precursors: | 25% |
| Lymphocytes: | 16% |
| Plasma cells: | 4% |
Figure 1: Post-operative day 4 bone marrow aspiration and biopsy findings. A) Bone marrow aspirate with spectrum of left-shifted myeloid cells, including promyelocytes, myelocytes, and metamyelocytes. B) Bone marrow aspirate with spectrum of left-shifted myeloid cells and admixed eosinophils. (A and B: Wright Giemsa stain; 1,000x original magnification). C) Bone marrow core biopsy showing 50% cellular marrow. (Hematoxylin and eosin stain; 40x original magnification). D) Bone marrow biopsy with myeloperoxidase immunohistochemistry stain demonstrating granulocyte precursors. (100x original magnification).
The patient was monitored closely with complete blood counts and a prolonged period of agranulocytosis was documented. On postoperative day 21, the white blood cell count was 3.65 K/uL with 0% neutrophils. However, on a post-operative day, 22 white blood cell count was 5.27 K/uL with 32% neutrophils and an absolute neutrophil count of 1.81 K/uL. Post-operative day 24 white blood cell count was 7.74 K/uL with 50% neutrophils and an absolute neutrophil count of 3.83 K/uL. The clinical course of the agranulocytosis is summarized in Table 2. During this prolonged period of agranulocytosis, the patient had intermittent fevers which were treated with a variety of antibiotics including vancomycin, cefepime, minocycline, and Levaquin. The patient was receiving cefepime at the time her neutrophil count began rising. After the onset of the agranulocytosis, it was noted that the patient had had a knee replacement operation 35 months previously. During that operation, the patient also received 2 grams of intravenous cefazolin.
| Table 2: Clinical Course of agranulocytosis. | |||
| Peripheral blood | WBC (k/uL) | % Neutrophils | ANC |
| Pre-operative | 6.8 | 64 | 4.3 |
| Post-operative day 1 | 1.93 | 0 | 0 |
| Post-operative day 21 | 3.65 | 0 | 0 |
| Post-operative day 22 | 5.27 | 32 | 1.81 |
| Post-operative day 24 | 7.74 | 50 | 3.83 |
| WBC: White Blood Cell Count; ANC: Absolute Neutrophil Count | |||
Cephalosporin-associated neutropenia and agranu-locytosis are well-known adverse reactions to these medications [6]. However, there are unusual features demonstrated by this case. In most cases of cephalosporin-associated neutropenia, there has been an extended course of antibiotics. The average time of drug exposure to the development of neutropenia has been cited as between 1 and 6 months [2-4]. Our patient received a single intravenous dose of cefazolin 35 months prior to the second dose which resulted in agranulocytosis. Outside of these 2 intraoperative intravenous infusions of cefazolin, the patient never had exposure to this drug. This suggests that in a susceptible host that a single high-dose intravenous infusion of this antibiotic is sufficient to sensitize the host to form drug-dependent anti-neutrophil antibodies. In addition, the rapid onset of agranulocytosis in our patient suggests that these antibodies can persist for at least 35 months, as that is the period of time between the 2 intravenous infusions of cefazolin in this patient. The median time to recovery of neutrophil counts in cases of cephalosporin-associated neutropenia/agranulocytosis is reported to be in the 2-7 day range, although there are reports with cases extending the length of time to 18 days [7]. In addition, the average time for full recovery of neutrophil count for all drugs implicated in this adverse reaction has been cited as 8 days with a range of up to 24 days [1]. The time to recovery in the patient we report was clearly documented at 21 days, which is a prolonged recovery period, particularly given the reported half-live of cefazolin, the drug implicated as the cause of the agranulocytosis, is only 1.6 hours.
The pathogenesis of drug-induced neutropenia and agranulocytosis have been well investigated, however, there has never been a clear explanation for the cases with a prolonged recovery time to normal circulating neutrophil levels. Both immunologic mechanisms leading to neutrophil destruction and toxic suppressive effects on the bone marrow have been postulated as pathogenetic mechanisms for drug-induced neutropenia [2-4]. It has also been suggested that when there is delayed recovery from neutropenia that this may indicate marrow toxicity and maturation arrest [7]. The bone marrow biopsy at day 4 showed a left-shifted marrow, but not a complete maturation arrest, with maturation arrest defined as an arrest of maturation of the granulocytic series at the progranulocyte stage [2]. The marrow cellularity was normal to slightly hypercellular for the patient’s age (50%). In addition, there was not an arrest at the progranulocyte stage of differentiation. This supports the interpretation that the absence of circulating neutrophils was not due to a production deficiency.
Most of the evidence supports immunologic destruction of neutrophils rather than a production arrest. Early evidence for immune-mediated destruction of neutrophils came from a remarkable transfusion experiment that could not be repeated today. The investigators took serum from a patient with pyramidon (aminophenazone)-induced neutropenia at the nadir of the neutrophil count and transfused it into 2 normal subjects. In these hosts the white blood cell count fell from 5.0 to 0.8 K/uL and 8.4 to 1.7 K/uL in the first 40 minutes, followed by a rapid recovery at 4 hours. Control transfusions caused no drop in the white blood cell counts. Two weeks later, these 2 normal subjects took a one-week course of the pyramidon and there was no change in the white blood cell count [10]. In an investigation of penicillin-induced neutropenia, complement-fixing IgG penicillin antibodies reacting with granulocytes in the presence of penicillin were detected [8]. Additional evidence for an immune-mediated process includes the additional clinical features of these reactions. 1) There needs to be prior sensitization to the drug or there needs to be prolonged administration of the drug so that there is sufficient time for a hypersensitivity reaction to occur. 2) There is a rapid recurrence of the neutropenia on rechallenge with the sensitizing drug. 3) The appearance of eosinophilia in association with these reactions. 4) The association of certain HLA types with idiosyncratic drug reactions [4].
In our case, there was a prior intravenous administration of cefazolin which we believe served as the sensitizing event. Given the body of evidence on similar drug-induced neutropenia cases, we believe that a drug-dependent anti-neutrophil antibody was responsible for the agranulocytosis. We believe there is an explanation for the prolonged recovery period. Although the half-life of cefazolin is only 1.6 hours, this drug is known to bind to albumen [11,12]. Albumen has a half-life of 19 days [13]. However, the binding to albumen is reversible and cefazolin can be displaced from the albumen by bilirubin, fatty acids, and a number of drugs [11]. It is known that cephalosporins, like penicillin, can bind covalently to neutrophils and form a hapten structure on the surface of the neutrophil which in an idiosyncratic setting can be immunogenic and lead to the production of an immune reaction with subsequent formation of drug-dependent anti-neutrophil antibodies [3,6,7]. We suggest that in a competitive binding setting that the albumen-associated cefazolin will strongly prefer binding to neutrophils. Given the long half-life of albumen (19 days), this would explain the presence of the drug available for binding to neutrophils for an extended period of time and would account for the 21 day recovery period seen in our case.
The final question revolves around the method of neutrophil destruction. In an examination of penicillin-associated neutropenia, it has been suggested that the drug-dependent antibodies cause agglutination of neutrophils with subsequent sequestration in a variety of sites including the spleen, liver, and lung [8]. A second hypothesis is that since these drug-dependent antibodies are IgG complement-fixing antibodies, it is complement fixation and activation that results in neutrophil lysis. In this regard, it is interesting to note a report of intravenous immunoglobin therapy leading to a short-term reversal of drug-induced neutropenia [14]. Intravenous immunoglobulin therapy in the treatment of autoimmune and inflammatory diseases has multiple proposed pathophysiologic mechanisms [15,16]. Complement fixing immunocomplexes result in the production of membrane attack complexes, and it has been proposed that the anti-inflammatory activity of intravenous immunoglobulin is at least partly mediated by its ability to prevent the formation of membrane attack complexes [15,16]. This would lend some support to the hypothesis that rather than agglutination of the neutrophils and subsequent removal by the reticuloendothelial system, neutrophil destruction is mediated by a neutrophil membrane attached immune complex activation of complement and formation of a membrane attack complex with subsequent cell lysis.
In summary, we report a case of postoperative agranu-locytosis secondary to cefazolin which had a prolonged recovery time to normal neutrophil levels. The case was remarkable also for the method of sensitization to the drug cefazolin. There was only a single prior intravenous administration of this drug, 35 months previous to the agranulocytosis-causing second dose of cefazolin, which served as the sensitizing event. We have proposed an explanation of the prolonged period for recovery which relies on the presence of drug-dependent anti-neutrophil antibodies, and which explains the prolonged presence of the drug cefazolin by invoking its known binding/attachment to albumen and postulating a competitive binding scenario which is greatly in favor of binding to sites on the neutrophil membrane, as opposed to the albumen binding.
Subsequent complement fixation and activation with the formation of a membrane attack complex results in neutrophil lysis.
- Andersohn F, Konzen C, Garbe E. Systemic review: Agranulocytosis induced by nonchemotherapy drugs. Ann Internal Med 2007; 146: 657-665. PubMed: https://pubmed.ncbi.nlm.nih.gov/17470834/
- Uetrecht J, Naisbitz DJ. Idiosyncratic adverse drug reactions: Current concepts. Pharmacol Rev. 2013; 65: 779-808. PubMed: https://pubmed.ncbi.nlm.nih.gov/23476052/
- Curtis BR. Non-chemotherapy drugs-induced neutropenia: Key points to manage the challenges. Hematology Am Soc Hematol Educ Program. 2017; 2017: 187-193. PubMed: https://pubmed.ncbi.nlm.nih.gov/29222255/
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis-infrequent but dangerous. Fronti Pharmacol. 2021; 12: 1-13. PubMed: https://pubmed.ncbi.nlm.nih.gov/34483939/
- Smith CR. Cefotaxime and cephalosporins: Adverse reactions in perspective. Rev Infect Dis. 1982; 4: S481-S487. PubMed: https://pubmed.ncbi.nlm.nih.gov/6294802/
- Murphy MF, Metcalfe P, Grint PCA, et al. Cephalosporin-induced immune neutropenia. Br J Haem. 1985; 59: 9-14. PubMed: https://pubmed.ncbi.nlm.nih.gov/3882133/
- Cimino C, Allos BM, Phillips EJ. A review of beta-lactam-associated neutropenia and implications for cross-reactivity. Ann Pharm. 2021; 55: 1037-1049. PubMed: https://pubmed.ncbi.nlm.nih.gov/33215507/
- Murphy MF, Riordan T, Minchinton RM, et al. Demonstration of an immune- mediated mechanism of penicillin-induced neutropenia and thrombocytopenia. Br J Haem. 1982; 55: 155-160. PubMed: https://pubmed.ncbi.nlm.nih.gov/6882683/
- Uy N, Thiagarajan P, Musher DM. Cephalosporin site chain idiosyncracies: A case report of ceftriaxone-induced agranulocytosis and review of literature. Open Forum Infect Dis. 2015; 2: ofv007. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4438888/
- Moeschlin S, Wagner K. Agranulocytosis due to the occurrence of leukocyte- agglutinins. Acta Haem. 1952; 8: 29-41. PubMed: https://pubmed.ncbi.nlm.nih.gov/12985212/
- Decroix MO, Zini R, Chaumeil JC, Tillement JP. Cefazolin serum protein binding and its inhibition by bilirubin, fatty acids and other drugs. Biochem Pharm. 1988; 37: 2807-2813. PubMed: https://pubmed.ncbi.nlm.nih.gov/3395358/
- Vella-Brincat JWA, Begg EJ, Kirkpatrick CMJ, Zhang M, Chambers ST, et al. Protein binding of cefazolin is saturable in vivo both between and within patients. Br J Cin Pharm. 2007; 63: 753-757. PubMed: https://pubmed.ncbi.nlm.nih.gov/17223858/
- Zorzi A, Linciano S, Angelini A. Non-covalently albumin-binding ligands for extending the circulating half-life of small biotherapeutics. Med Chem Commun. 2019; 10: 1068-1081. PubMed: https://pubmed.ncbi.nlm.nih.gov/31391879/
- Getta B, Ponnian G, Ling S. Intravenous immunoglobulin induces short-term reversal of drug-induced autoimmune neutropenia." Letter to editor. Transfus Med. 2015; 25: 347-348. PubMed: https://pubmed.ncbi.nlm.nih.gov/26192766/
- Jacob S, Rajabally YA. Currant proposed mechanisms of action of intravenous immunoglobulins in inflammatory neuropathies. Curr Neuropharmacol. 2009; 7: 337-342. PubMed: https://pubmed.ncbi.nlm.nih.gov/20514213/
- Norris PAA, Kaur G, Lazarus AH. New insight into intravenous immunoglobulin mechanisms and alternatives in autoimmune and inflammatory diseases. Curr Opin Hematol. 2020; 27: 392-398. PubMed: https://pubmed.ncbi.nlm.nih.gov/32868670/